Work Drug Safe
Work Drug Safe
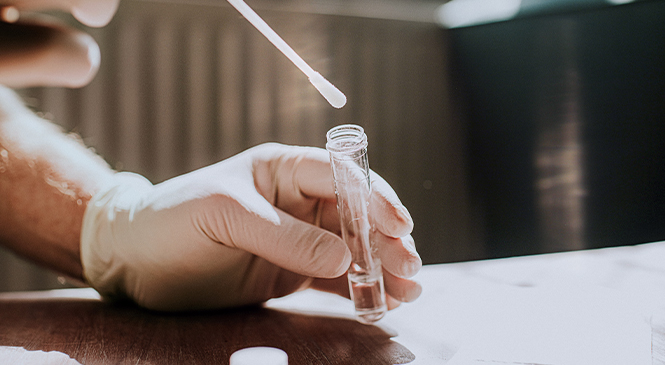
The Substance Abuse & Mental Health Services Administration’s (SAMHSA’s) released mandatory guidelines for oral fluid testing (OFMG) in 2019.1 Recently the U.S. Department of Transportation (DOT) proposed an amendment to the transportation industry drug testing program regulations to include oral fluid testing.2 This movement to oral fluid as an option for regulated drug testing has many providers and buyers of drug testing interested in learning more about lab-based oral fluid drug testing and how oral fluid testing compares to urine testing.
“Lab-based oral fluid testing has been used and accepted in the judicial system for a long time. The reason for acceptance is sound scientific technology behind lab-based oral fluid testing."
“Oral fluid drug testing is based on the same scientific principles and conducted using the same technology as urine. It is scientifically accurate."
1. Mandatory Guidelines for Federal Workplace Drug Testing Programs-Oral/Fluid. Federal Register, The Department of Health and Human Services, 25 Oct. 2019, https://www.federalregister.gov/documents/2019/10/25/2019-22684/mandatory-guidelines-for-federal-workplace-drug-testing-programs-oralfluid.
2. Department of Transportation Office of the Secretary Proposed Rule 87 FR 11156 https://www.federalregister.gov/documents/2022/02/28/2022-02364/procedures-for-transportation-workplace-drug-and-alcohol-testing-programs-addition-of-oral-fluid
3. Mandatory Guidelines for Federal Workplace Drug Testing Programs-Oral/Fluid.” Federal Register, The Department of Health and Human Services, 25 Oct. 2019, https://www.federalregister.gov/documents/2019/10/25/2019-22684/mandatory-guidelines-for-federal-workplace-drug-testing-programs-oralfluid.
4. See Medina County State of Ohio v. Albert Hoelscher , Imperial Oil Ltd. v. Communications, Energy & Paperworkers Union of Canada, Medina County v. Hixon, or Family Court of the State of New York
5. Full list of conditions can be found in the “Mandatory Guidelines for Federal Workplace Drug Testing Programs-Oral/Fluid) Section 7.2 (What are the requirements for an oral fluid collection device?).
6. Mandatory Guidelines.
7. Mandatory Guidelines for Federal Workplace Drug Testing Programs-Oral/Fluid.” Federal Register, The Department of Health and Human Services, 25 Oct. 2019, https://www.federalregister.gov/documents/2019/10/25/2019-22684/mandatory-guidelines-for-federal-workplace-drug-testing-programs-oralfluid.
8. See “Current List of HHS-Certified Laboratories and Instrumented Initial Testing Facilities which Meet minimum Standards to Engage in Urine and Oral Fluid Drug Testing for Federal Agencies” February 1, 2022. https://www.govinfo.gov/content/pkg/FR-2022-02-01/pdf/2022-01990.pdf.
9. See “Current List of HHS-Certified Laboratories and Instrumented Initial Testing Facilities which Meet minimum Standards to Engage in Urine and Oral Fluid Drug Testing for Federal Agencies” February 1, 2022. https://www.govinfo.gov/content/pkg/FR-2022-02-01/pdf/2022-01990.pdf.
Drug Safe Workplace
©2024 Abbott. All rights reserved. Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.
This website is governed by applicable U.S. laws and governmental regulations. The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage.
Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy.
Abbott - A Global Leader in Toxicology.
Links which take you out of Abbott worldwide websites are not under the control of Abbott, and Abbott is not responsible for the contents of any such site or any further links from such site. Abbott is providing these links to you only as a convenience, and the inclusion of any link does not imply endorsement of the linked site by Abbott. The website that you have requested also may not be optimised for your screen size.